Carvedilol
Observational Study
Carvedilol as a potential Anti-Seizure Medication
Your doctor may tell you your child may be a candidate to try carvedilol to reduce seizures due to KCNT1-related epileptic encephalopathy.
Why This Study?
Purpose of this Study
Potential Risks and Benefits
How do I know if I qualify for this Study?
How to Get Started
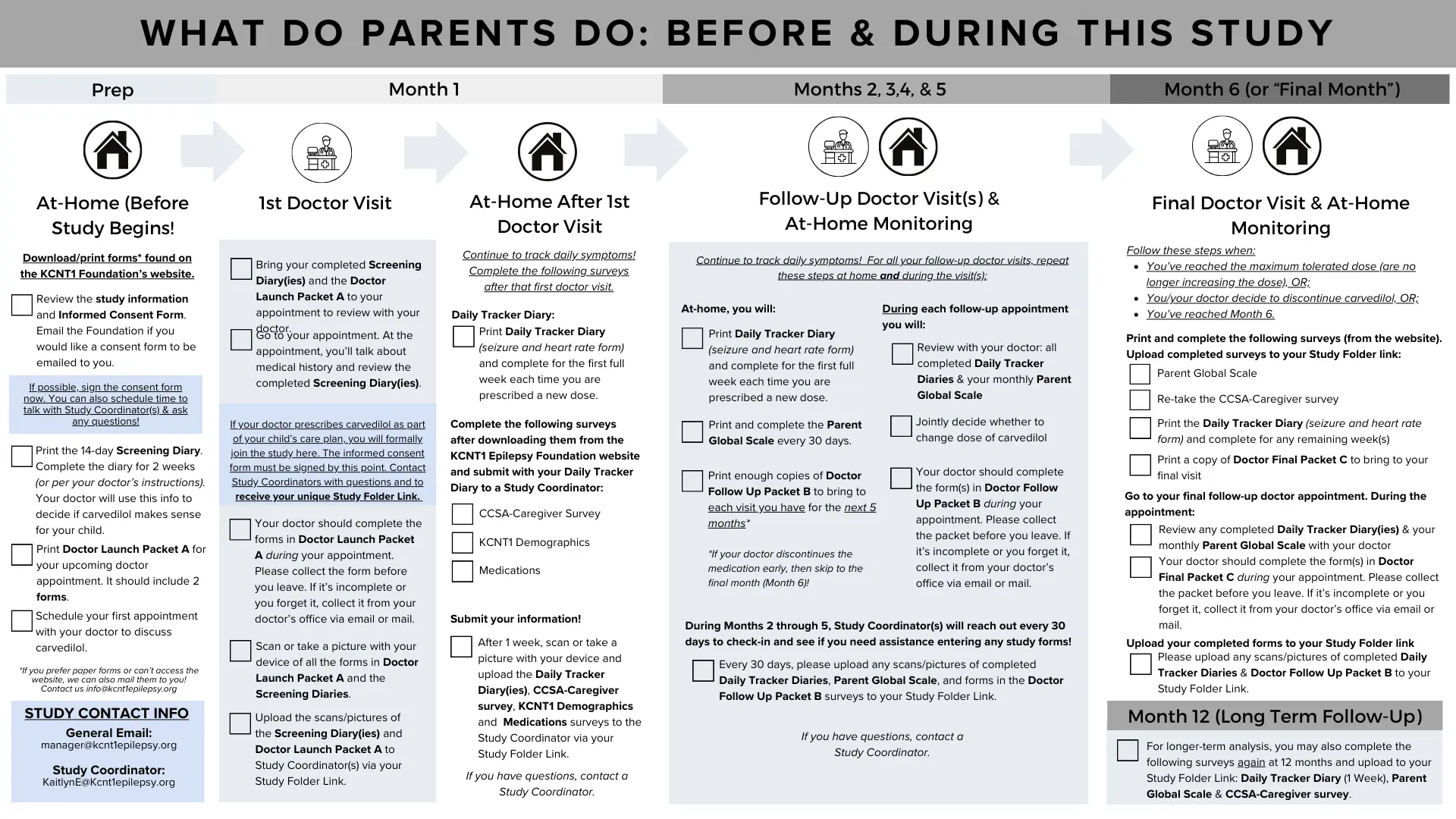
What do I bring to my Doctor visits?
Downloadable Forms for Parents and Doctors
In this observational study, your child’s doctor will prescribe carvedilol for your child, and you and your doctor will collect information about its effects and your child’s response to it. This will help your doctor determine if carvedilol effectively changes the signs or symptoms of KCNT1-related epileptic encephalopathy, specifically seizure activity.
The decision for the prescription of carvedilol for on- or off-label use lies solely with your doctor, following a discussion involving its risks and benefits with the patient and his or her family.
Caregivers can learn more about the steps required in this study in this downloadable handout.
Clinicians can learn more about the steps in this study in this handout.
The study forms can be downloaded below, or they can be mailed to you. If your doctor prescribes carvedilol and you wish to participate in the study, email the KCNT1 Epilepsy Foundation and they will provide a link to an online storage drive for you to upload your completed forms throughout the study.
Informed Consent
Screening Diary
Daily Tracker Diary
CCSA-Caregiver Survey
Parent Global Scale
Medication Survey
KCNT1 Demographics Survey
Printable Packet A
Printable Packet B
Printable Packet C