Your Participation Powers KCNT1 Research and Treatments
Every registry entry, survey, and study moves us closer to better care and cures!


Understanding Clinical Research
Clinical research is medical research that involves people. It helps scientists answer important questions and develop new treatments, devices, or ways to improve care.
Every study is different, and it’s important to understand the type of study before you decide to participate.
Two Main Types of Clinical Research

Observational Studies
- Researchers collect information about participants but do not assign a new treatment or intervention.
- Examples: registries, natural history studies, biomarker studies, surveys, reviewing EEGs and medical records, or collecting blood or other samples.
- Why they matter: They create baseline data needed to design and launch clinical trials. Families can usually participate in multiple observational studies at once.

Clinical Trials (Interventional Studies)
- Researchers test a new treatment, device, or procedure to see if it is safe and effective.
- In a clinical trial, participants are randomly assigned to receive either the investigational treatment, a medical device, or something to compare it against—such as the current standard of care or a placebo.
- Trials go through phases (I–III) to answer questions about safety, effectiveness, and comparisons to current options.
- Why trials matter: They provide the evidence regulators like the FDA need to approve new therapies.
Why Your Participation Matters
Your story, data, and questions shape the future of KCNT1 treatments. In rare diseases like KCNT1-related disorders, families are often the first—and sometimes the only—source of critical insights.
Every survey completed, sample shared, and trial joined moves us closer to better care and cures.

How Families Can Help Solve the Puzzle
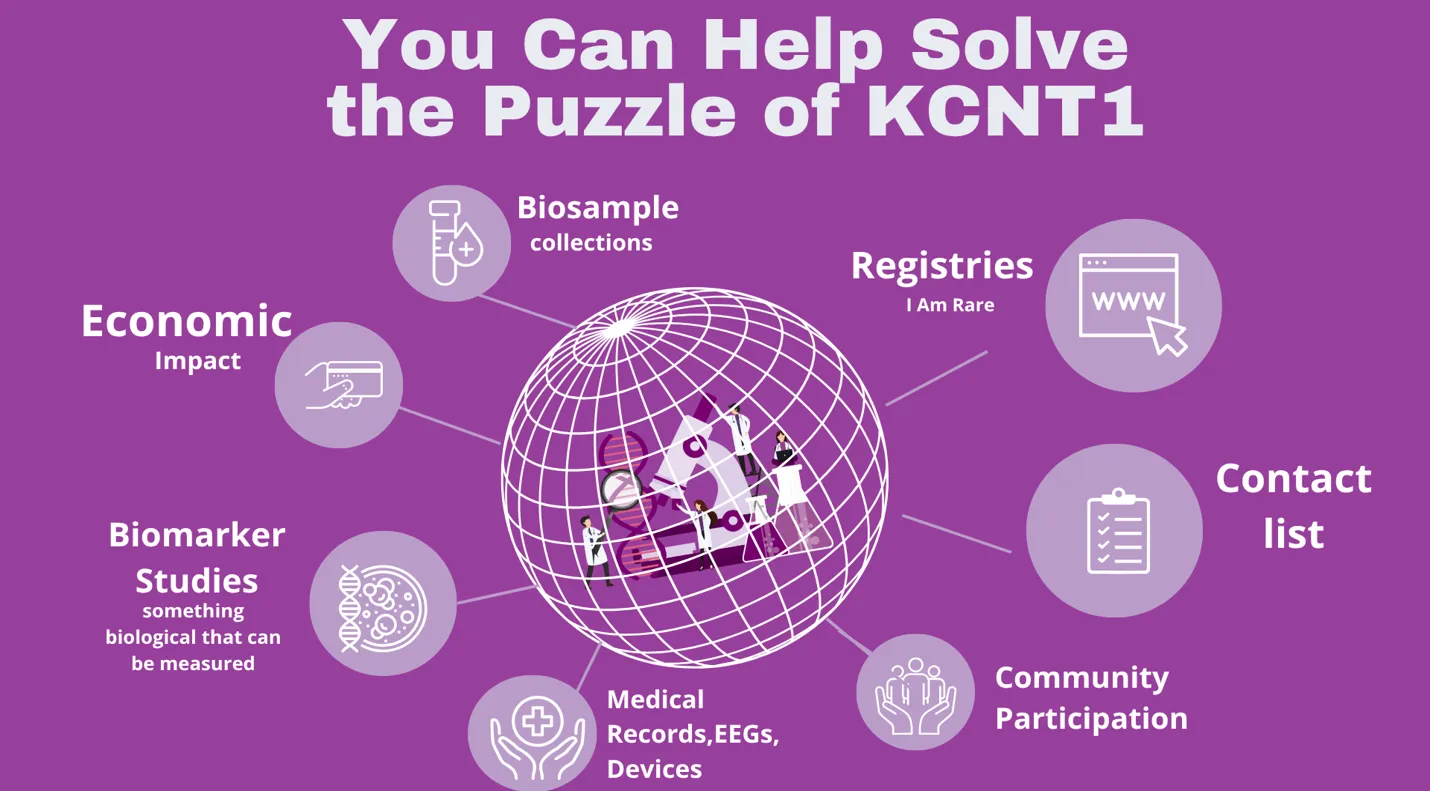
Participation in research doesn’t always mean trying a new drug. Families can make an impact in many ways:
Share information through Observational studies
Enroll in the KCNT1 International Registry, complete symptom and quality-of-life surveys, or consent to securely share medical records, blood and tissue samples, and EEGs.
Volunteer for clinical studies or trials
These test whether a new treatment works, what side effects it may cause, and how it compares to current therapies.
Current Observational Studies
KCNT1 International Registry (coming soon)
Who can participate: Anyone with a KCNT1 variant
What’s involved: Series of online surveys (Coming soon)
Citizen Health Natural History Study
Danish ADSHE EMR Study
Blood and Tissue Donation – Biosample Repository
Biomarker Study (coming soon)
EEG Collection (coming soon)
KCNT1-Specific Clinical Trials
Currently there are no approved clinical trials designed for KCNT1, but several therapeutics are in the pipeline. Announcements are expected soon.
By joining a trial, you are not only trying something new for your child—you are contributing to research that could change the future for others like them. It’s a deeply personal choice, and this guide is here to help you understand all aspects of that decision.
Your Role Is More Than Just a Participant As a parent, you know your child best. Your observations, questions, and feedback are incredibly valuable to the research team. You are a partner in the process.
You’ll help collect data—like seizure logs, behavior changes, or improvements in communication—that help researchers understand whether a treatment is really making a difference.
Actio Biosciences
Therapy: ABS-1230
Phase: Phase 1 – The FDA approved testing in healthy adults. Trials are expected to enroll in 2026.
Class: Small molecule
Target: KCNT1
Delivery: Oral
Servier
Therapy: ASO
Class: ASO
Target: KCNT1
Delivery: Intrathecal
Atalanta
Therapy: ATL-201
Phase: IND-enabling
Class: di-siRNA
Target: KCNT1
Delivery: Intrathecal
Undisclosed
Therapy: Small molecule or ASO
Phase: Preclinical
Target: KCNT1
Delivery: Oral or Intrathecal
Other Clinical Trials
Lundbeck
Therapy: Bexicaserin
Phase: Phase 3
Class: Small molecule
Target: 5-HT2C
Delivery: Oral
Status: Enrolling
Frequently Asked Questions about Clinical Trials:
Dive deeper into common questions about participating in clinical research and trials.
Can I participate in more than one study at the same time?
You can often participate in multiple observational studies because they don’t involve experimental therapies. For clinical trials, you must ask the trial’s Primary Investigator, as some studies prohibit participation in another trial simultaneously to ensure data integrity.
Who is eligible to participate in a clinical trial?
If I am eligible for a trial, how do I enroll?
What if I don’t qualify for a clinical trial?
Safety & Ethics: How do I know if a study is safe?
Safety & Ethics: What are my rights and responsibilities?
Safety & Ethics: Can I share my experience publicly?
What to Expect: What is informed consent?
What to Expect: Will my medical care change?
What to Expect: What happens during a clinical trial?
What to Expect: How long do clinical trials last?
Finding Trials: Where can I find out about clinical trials?
Finding Trials: Can I join if I live outside the U.S.?
Finding Trials: Do studies cost money to join?

Finding Trials
- Visit www.ClinicalTrials.gov for a searchable database of U.S. and international trials.
- Check the KCNT1 Trials & Studies Portal for studies specific to KCNT1.

Disclaimer
We gratefully acknowledge many organizations for educational resources we have provided here: SCN2aFamilies, Global Genes, Dravet Syndrome Foundation, International Rett Syndrome Foundation, Angelman Clinical Trials, LGS Foundation, NIH, HHS, FDA, and others.



