
CLINICAL TRIALS ARE COMING!
KCNT1 Therapeutic Development
News & Updates
The most up-to-date information on research
to deliver treatments and cures for KCNT1-related epilepsy.
At the KCNT1 Epilepsy Foundation, we’re committed to driving research forward through parent participation in both clinical trials and observational studies. By contributing real-world data and joining studies when possible, families play a powerful role in accelerating the path to new treatments.
We’re also here to help our community understand what it means to participate in a clinical trial. Clinical trials are research studies performed in people that test new ways to detect, diagnose, treat, or prevent disease. We will connect our community members with trial opportunities as they become available and provide clear, unbiased educational information to support informed decision-making. Please note: we do not provide medical advice and are not affiliated with or endorsing any one life science company.
More than 15 companies and academic labs are currently working on therapeutics specifically aimed at treating KCNT1-related epilepsy. This is a major breakthrough—because current, generic anti-seizure medications do not target the dysfunctional KCNT1-encoded potassium channel in the brain and have shown limited or no benefit in most affected individuals. We will be highlighting therapies designed to act directly on KCNT1, many of which are first-in-class approaches.
Scroll down to learn more about the companies investing in KCNT1 research, the scientific strategies they’re testing, and where each program stands in the research and development pipeline. You’ll also find guidance on how to become “clinical trial ready”—a key step in bringing new treatment options to life for our community.
THERAPEUTIC DEVELOPMENT PIPELINE
The pathway to developing a therapeutic can be divided into five stages: discovery and development, preclinical research, clinical research, drug review, and post-market safety monitoring. So far, therapeutics for KCNT1 have been developed through the discovery and preclinical phases, and have yet to progress to clinical trial.
Stage 1: Discovery & Development
Several companies and labs are in the discovery and development stage which spans basic research in the lab to animal model research. During this step a potential therapeutic is identified and tested in many ways in the lab to show that it works (called efficacy), and a most promising version of the therapeutic (often called the lead candidate) is chosen for next stages. This stage may involve 5-10 years of testing.
Stage 2: Preclinical Research & Development
After a lead candidate has been selected in the discovery stage, the candidate therapeutic must be tested more carefully and strenuously for safety and efficacy in cell culture and animal models. These tests follow regulatory agency mandated requirements. This stage can last 3-5 years and if successful, results in an Investigational New Drug Application (IND) application to the Food and Drug Administration (FDA), which is a request to begin clinical trials. To date, no KCNT1 therapeutic has moved from the preclinical phase to the clinical phase, though we hope clinical trials may initiate in late 2025.
Stage 3: Clinical Trials – Phase 1, 2, and 3
Once an Investigational New Drug (IND) application is approved by the FDA, the therapeutic enters the clinical trial phase, which involves testing in humans. These trials are conducted in three progressive phases.
- Phase 1 involves a small group of healthy volunteers or affected individuals and is primarily focused on determining safety, dosage range, and side effects.
- Phase 2 expands the testing to a larger group of affected individuals to evaluate efficacy and continue monitoring safety.
- Phase 3 includes even larger populations—often hundreds of patients across multiple clinical sites—to confirm effectiveness, monitor side effects, and compare the drug to standard or placebo treatments.
For rare diseases like KCNT1-related epilepsy, patient recruitment is challenging and may require international collaboration and innovative trial designs. The full clinical trial process may span 5–7 years or longer depending on the availability of patients, trial design, and regulatory review. If successful, the company can then submit a New Drug Application (NDA) to the FDA for marketing approval.
Stage 4: FDA Review, Approval & Post-Market Monitoring (Phase 4)
If the Phase 3 results support the therapeutic’s safety and effectiveness, the FDA will review the NDA, which includes all data from laboratory, animal, and human studies. This regulatory review period typically takes 6–10 months. If approved, the therapeutic may be sold and prescribed to patients. However, the process doesn’t end here.
In Phase 4 (post-marketing surveillance), the therapeutic continues to be monitored for long-term safety, side effects, and effectiveness in the broader population. This stage is crucial for identifying rare adverse events that may not have appeared during earlier phases and may lead to updated warnings, label changes, or in rare cases, withdrawal from the market. For KCNT1, post-market studies could help identify long-term impacts on neurodevelopment or seizure burden and inform ongoing care strategies.
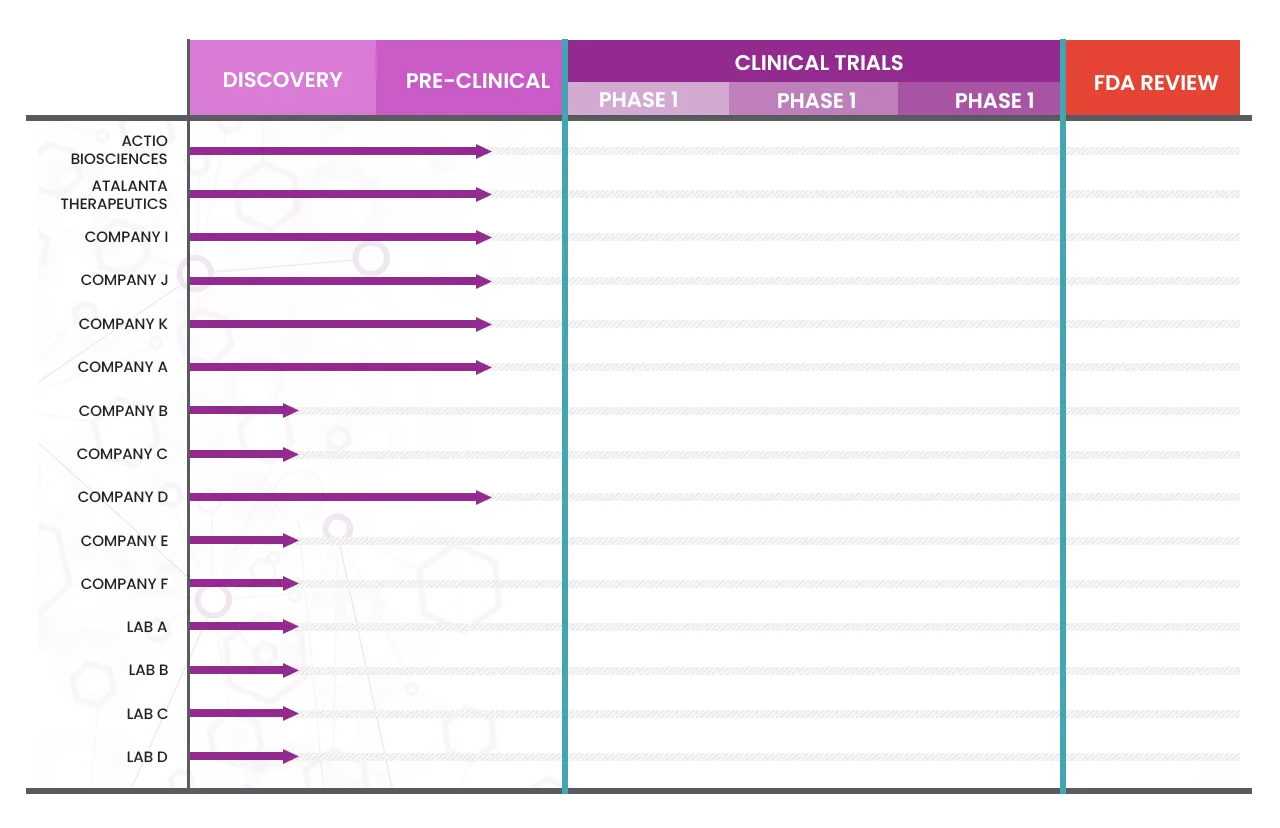
Currently there are no trials designed specifically for KCNT1 related epilepsy or related disorders but hopefully, will be coming in late 2025 or 2026. There are several companies who are testing treatments in their labs, so they are designated as pre-clinical.
INDs for 3 KCNT1-directed therapies should be filed in 2025:
1. ASO – Servier
2. Divalent siRNA – Atalanta Therapeutics
3. Small molecule – Actio Bio
More information is coming soon!
Clinical Trial Education Series
Empowering Parents to Navigate Clinical Trials with Clarity and Confidence
Course Overview
We are entering an exciting era in rare disease treatment, with many promising clinical trials on the horizon. For families, this means more options—but also more decisions. This self-paced curriculum helps you understand the clinical trial process, so you can work with your child’s doctor to decide whether a study is a good fit. If your child meets a trial’s inclusion and exclusion criteria, this course will help you feel confident in navigating next steps.
Module 1: Understanding the Science & Therapies
Duration: 1.5 hours
- What is gene therapy? Viral vs. non-viral delivery methods
- Basics of DNA/RNA, how gene therapy targets neurological disorders
- Small molecules and ASOs: how these therapies work
- From lab to patient: preclinical and clinical research explained
- Key challenges: durability, regulatory hurdles, and access
Module 2: The Clinical Trial Process
Module 3: Participating in a Trial
Watch our Webinar on the “Path to a Trial” on our YouTube channel. This presentation by Drs. Ali Rosenberg and Brad Bryan introduces the clinical development pipeline, the KCNT1 Epilepsy Foundation’s contributions to the clinical pipeline, a research and clinical trials overview, and what clinical trial readiness and our educational plan looks for the KCNT1 community. Important information includes overviews on the steps patients and families can take to be clinical trial ready and the role of caregivers in clinical trials. Future webinars will cover clinical trial basics, clinical trial preparation, addressing concerns, caregiver support, and the enrollment process.
Watch our Webinar on “Your Data: What We Know Today” on our YouTube channel. This presentation by Megan Wright and Dr. Brad Bryan shows what we’ve learned about our KCNT1 community by analyzing the data from our former Luna registry and the Citizen database, highlighting the importance of coming together to share electronic medical record data through these platforms. Megan shows data on how our community has grown and demographic information, and Brad shows what he’s gathered from the data about diagnoses, common procedures, medications, reasons for hospital admissions, and more.
Stay tuned for future updates on upcoming trials and webinars to teach you about everything you need to know to be ready!
Clinical Trials are Research Studies
A research study that measures the results of an intervention like a treatment or medication is called a clinical trial. It’s a study that tests (or tries out) an intervention — a potential drug, medical device, activity, or procedure — in people. They scientifically evaluate questions such as:
- Does this investigational drug work?
- Does it work better than another medicine already available?
- Does it cause any side effects?
- Are there any other benefits that could improve patient quality of life?
- Is there a new way to use an existing treatment?
Without volunteers to enroll, clinical trials cannot exist, and we would not be able to find new treatments. Families who participate in clinical trials are on the cutting edge of helping us to find better treatments and cures.
Should I Participate In A Clinical Trial?
The main purpose of clinical trials is to see how a new medical product works in people. It is important for people who are considering participation in a clinical trial to understand their role, as a “subject of research” and not as a patient.
Key points: While research subjects may get personal treatment benefit from participating in a clinical trial, they must understand that they:
- may not benefit from the clinical trial,
- may be exposed to unknown risks,
- are entering into a study that may be very different from the standard medical practices that they currently know
To make an informed decision about whether to participate or not in a clinical trial, people need to be informed about:
- what will be done to them,
- how the protocol (plan of research) works,
- what risks or discomforts they may experience,
- participation being a voluntary decision on their part.
Learn more here on things to consider before you participate in a trial.
KCNT1-Specific Trials
Clinical Trials For KCNT1-related Epilepsy
Currently, treatments are in development for KCNT1 but there are no trials available yet. There are several companies who are testing treatments in their labs, so they are designated as pre-clinical. More information is coming soon!
Clinical Trials For General Epilepsy or Other Syndromes
The following trials are not designed for KCNT1, however you might consider discussing them with your healthcare team.
Remember to read the details and discuss with your team, you may receive a placebo in some trials. Be sure to review the Informed Consent with your doctor.
Bexicaserin: Bexicaserin (LP352) is an oral, centrally acting 5-hydroxytryptamine 2C (5-HT2C) receptor superagonist with no observed impact on 5-HT2B and 5-HT2A receptor subtypes. It is being evaluated in a global Phase III clinical program (the DEEp Program). The FDA has granted Breakthrough Therapy Designation for bexicaserin for the treatment of seizures associated with Developmental and Epileptic Encephalopathies (DEEs) for patients two years of age and older. Bexicaserin is an investigational compound that is not approved for marketing by the FDA or any other regulatory authority.
To Learn More or Search for Clinical Trials
For more information about clinical trials, visit NIH, ClinicalTrials.gov.
https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
For information about clinical trials sponsored by private sources, contact:
http://www.centerwatch.com/
For information about clinical trials conducted in Europe, contact:
https://www.clinicaltrialsregister.eu/



